Topological Analysis of the Electron Density in the Carbonyl Complexes M(CO)8 (M = Ca, Sr, Ba)
Detalles de la Publicación
Revista: Organometallics 2020, 39, 1, 132-141
Autores: Juan F. Van der Maelen.
Factor de Impacto: 4,1
Resumen
The quantum theory of atoms in molecules
(QTAIM) has been applied to the recently synthesized
alkaline-earth cubic Oh-symmetric complexes Ca(CO)8 (1),
Sr(CO)8 (2), and Ba(CO)8 (3). Theoretical calculations
reveal that M−CO interactions in these complexes can be
properly described as highly polar bonds, showing some
features traditionally associated with transition-metal bonding,
although with noticeable differences, as well. In this sense,
δ(M−C) and δ(M···O) delocalization indexes for bonding
and nonbonding interactions, electron localization funcion (ELF) analyses, source function (SF) calculations, and the
interacting quantum atoms (IQA) approach, among other methodologies, produce results consistent with interactions
dominated by electrostatics between the CO ligands and alkaline-earth metals, with an increasing degree of covalency on going
from 1 to 3 and without any significant π-back-donation.
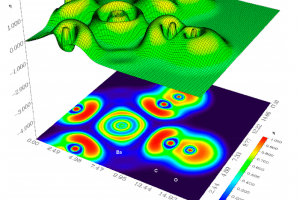
- Electron localization function projection on a C−Ba−C plane for the Ba(CO)8 complex (distances in bohrs).